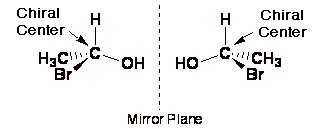
Cofty - that is correct. The left and right handed come from the idea of non-superimposable mirror images
Abiogenesis - moving on from the Urey Miller Experiment
by cantleave 37 Replies latest watchtower beliefs
-
-
cofty
Thanks.
The question of the origin of life has been raised so many times on the forum. It's great to see the subject being addressed with up to date research.
-
cantleave
For those that take the time to read the full paper the chemistry is awesome this hypothesis even gives a model for the evolution of the Krebs cycle.
Edit - if you read the full paper you will see why I ridicule the likes of "Answers in Genesis".....
-
70wksfyrs
Hey so my theory was close to Martin and Russell. Martin and Russell’s hypothesis state that the formation of the correct enantiomers starts at the RNA stage. This is cutting edge chemistry. I love it.
I wonder if their theory doesn’t pan out, they may in the future try the synthesis at the amino acid stereoisomer stage.
Cantleave - it was my understanding that it actually is possible to synthesise chemicals in the lab at a smaller molecular level in organic stereoisomers. I am sure I learned that the thalidomide drug was banned because in the 70's they did not separate them. They discovered the drug caused the deformities and then designed a new drug with the correct amount of L and D enantiomers.
It is my understanding that a racemic mixture occurs only with glycerides, but even with the racemic mixture they can be separated with polarisation techniques. But I have only measured polarity, never actually separated enantiomers. Hence I do not have any experience to draw on.
Please correct me if I am wrong, you are clearly more knowledgeable than I, so you may well teach me a thing or two. This is better than whining on my other thread.
-
70wksfyrs
cofty- wow your humility is refreshing. I really respect that you asked that question in the public domain. Kudos to you. It depends at which stage of education you are as to the terms used. Chemistry is a linier subject. I tend to use terms from all levels when I discuss chemistry. The term that pop's into my head.
Sorry I am poor a writing in a coherent and understandable manner, thankfully cantleave is a better writer than me.
You also asked
"20 left-handed ones are found in organic proteins?"
Yeah that is kind of right. Personally I would term it something like this
In nature about 20% of D-enantiomers are found in amino acids.
First of all, there are no hard and fast exact numbers it’s different in every case and secondly, the D-enantiomer is actually the right handed amino acid, and yes they are found in proteins. As cantleave said in synthesised sugars (glycerides) it’s 50/50 they are also organic.
Thanks again for putting the whole question on the board, I am sure cantleave will back me up on this one, you could have sent him a PM, but you let us all see what your question was.
-
70wksfyrs
Hey boys on this thread,
I am assuming you are all men due to my experience, so please refresh me if I am wrong. Now I can ignore the second part of this thread, but because cofty was so wonderfully humble, and this is a JW site, and you saw my whining thread, lets talk about CREATION. We are after all on a JW website!
I am probably the only girl here, and my ex is a pig so I don’t like men much and I can generalise from time to time. You boys have been great though. I am not ready yet to disclose what I believe. Cantleave already knows, so please keep this confidential if you remember. Maybe that’s given the game away but I am admitting to nothing just yet. Plausible deniability!!!!!!
In my view and the circles of people I have had personal experience with, scientists, chemistry mainly do not identify themselves as creationists and evolutionists. As a JW we have to preach 24/7 and I was a sucked in company girl, loved the GB very much. So I talked about God a lot, I found the majority of atheist scientists shut me down, because there were just not interested in talking about God or creation. I found that believers were so, because of background mainly not really because chemistry proved them right. One guy actually gave me a bible.
The only people that tried to prove me wrong with science, were atheists on the mino, students especially. They fell flat on their face when they started getting dogmatic, I got my knowledge out. I must also say I didn't use my knowledge with all atheists on the doors, as I was a good JW and wanted to win them over and not humiliate them.
As to what I believe now that I am Df'd, and leading a double life (cos I am still in) that is for a new thread.
So boys be kind, I have responded a little to the creationist part of the thread. I am after all a weaker vessel, and I was upset this morning. So please be kind.
70wks......
-
Phizzy
Dear 70wksfyrs, welcome to this board, and I certainly hope that your plea to be treated gently and with respect is adhered to, but I also hope it is unnecessary.
We just love intelligent people here, who are prepared to state their point of view, and will engage in debate, with due respect to other contributors. You tick all the boxes !
And thank you for your contributions to this thread, I am a simple Country Bumpkin compared to you and Cantleave in this area, but what a fascinating insight you, and he, and Cofty by asking the right question, have given us !
Science just makes my heart sing ! it has no bull****, just findings and conclusions for us to make of what we will, and the door left open for more discovery and more understanding. How different from the WT !
Please, you 70, and Cantleave, and dear invaluable Cofty, keep the "Science for Dummies like Phizzy" coming, we need it !
-
cofty
Thanks for your input 70wks. I hope your day is getting better.
I am a complete amateur in science but I love learning and trying to help make intersting things available to others.
I think science has great potential to help some JWs see the flaws in the deeply anti-scientific Watchtower.
-
jgnat
70wks, you've got it going on. I predict a brighter future ahead.
-
cantleave
Cantleave - it was my understanding that it actually is possible to synthesise chemicals in the lab at a smaller molecular level in organic stereoisomers.
I am not sure what you mean by that. In organic chemistry simplest stereoisomers are assymetric tetrahedral carbon compounds such as...
Whenever you have a carbon atom bonded to 4 different species you will have a chiral centre and as long as there are no planes of symmetry in the molecule that molecule will display stereo symmetry.
I am sure I learned that the thalidomide drug was banned because in the 70's they did not separate them. They discovered the drug caused the deformities and then designed a new drug with the correct amount of L and D enantiomers.
That's partially correct, Thalidomide has a chiral carbon atom and exists as 2 enantiomers. Initial tests sugessted that enantiomer was teratogenic while the other was therapeutic, tragically subsequent test showed that the entiomers can interconvert in-vivo so producing an enantiomerically pure preparation will not guarantee there will be no tetrogenic effects . The drug will never be used as an anti-morning sickness remedy but is being explored for other conditions.
http://www.nature.com/news/2010/100311/full/news.2010.117.html
It is my understanding that a racemic mixture occurs only with glycerides, but even with the racemic mixture they can be separated with polarisation techniques. But I have only measured polarity, never actually separated enantiomers. Hence I do not have any experience to draw on.
Racemic mixtures are common and I am certain that they are not unique to glycerides. A r acemic mixture is often formed when achiral substances are converted into chiral ones. An achiral substance in an achiral environment shows no preference to form one enantiomer over another.
You would think that separating enantiomers would be difficult since they show the same physical properties - boiling point etc. But as already acknowledged with Thalidomide they can display different chemical or bonding properties. This means resolving techniques or agents can be created to facillitate separation, these can chemical (e,g enzymatic reactions). They can also be separted by physical techniques like chiral chromotography, countercurrent fractionation and liquid membrane technology .