General information in how paleontologists and associated applicable sciences are used to date fossils. ........ no faith involved or needed
Determining the numerical age of rocks and fossils
Unlike relative dating methods,
absolute dating methods provide chronological estimates of the age of certain geological materials associated with fossils, and even direct age measurements of the fossil material itself. To establish the age of a rock or a fossil, researchers use some type of clock to determine the date it was formed. Geologists commonly use
radiometric dating methods, based on the natural
radioactive decay of certain elements such as potassium and carbon, as reliable clocks to date ancient events. Geologists also use other methods - such as
electron spin resonance and
thermoluminescence, which assess the effects of
radioactivity on the accumulation of electrons in imperfections, or "traps," in the crystal structure of a mineral - to determine the age of the rocks or fossils.
All elements contain protons and neutrons, located in the atomic nucleus, and electrons that orbit around the nucleus (Figure 5a). In each element, the number of protons is constant while the number of neutrons and electrons can vary. Atoms of the same element but with different number of neutrons are called isotopes of that element. Each isotope is identified by its atomic mass, which is the number of protons plus neutrons. For example, the element carbon has six protons, but can have six, seven, or eight neutrons. Thus, carbon has three isotopes: carbon 12 (12C), carbon 13 (13C), and carbon 14 (14C) (Figure 5a).

Figure 5: Radioactive isotopes and how they decay through time.
(a) Carbon has three isotopes with different numbers of neutrons: carbon 12 (C12, 6 protons + 6 neutrons), carbon 13 (C13, 6 protons + 7 neutrons), and carbon 14 (C14, 6 protons + 8 neutrons). C12 and C13 are stable. The atomic nucleus in C14 is unstable making the isotope radioactive. Because it is unstable, occasionally C14 undergoes radioactive decay to become stable nitrogen (N14). (b) The radioactive atoms (parent isotopes) in any mineral decay over time into stable daughter isotopes. The amount of time it takes for half of the parent isotopes to decay into daughter isotopes is known as the half-life of the radioactive isotope.
© 2013
Nature Education All rights reserved.

Most isotopes found on Earth are generally stable and do not change. However some isotopes, like 14C, have an unstable nucleus and are radioactive. This means that occasionally the unstable isotope will change its number of protons, neutrons, or both. This change is called radioactive decay. For example, unstable 14C transforms to stable nitrogen (14N). The atomic nucleus that decays is called the parent isotope. The product of the decay is called the daughter isotope. In the example, 14C is the parent and 14N is the daughter.
Some minerals in rocks and organic matter (e.g., wood, bones, and shells) can contain radioactive isotopes. The abundances of parent and daughter isotopes in a sample can be measured and used to determine their age. This method is known as radiometric dating. Some commonly used dating methods are summarized in Table 1.
The rate of decay for many radioactive isotopes has been measured and does not change over time. Thus, each radioactive isotope has been decaying at the same rate since it was formed, ticking along regularly like a clock. For example, when potassium is incorporated into a mineral that forms when lava cools, there is no argon from previous decay (argon, a gas, escapes into the atmosphere while the lava is still molten). When that mineral forms and the rock cools enough that argon can no longer escape, the "radiometric clock" starts. Over time, the radioactive isotope of potassium decays slowly into stable argon, which accumulates in the mineral.
The amount of time that it takes for half of the parent isotope to decay into daughter isotopes is called the half-life of an isotope (Figure 5b). When the quantities of the parent and daughter isotopes are equal, one half-life has occurred. If the half life of an isotope is known, the abundance of the parent and daughter isotopes can be measured and the amount of time that has elapsed since the "radiometric clock" started can be calculated.
For example, if the measured abundance of 14C and 14N in a bone are equal, one half-life has passed and the bone is 5,730 years old (an amount equal to the half-life of 14C). If there is three times less 14C than 14N in the bone, two half lives have passed and the sample is 11,460 years old. However, if the bone is 70,000 years or older the amount of 14C left in the bone will be too small to measure accurately. Thus, radiocarbon dating is only useful for measuring things that were formed in the relatively recent geologic past. Luckily, there are methods, such as the commonly used potassium-argon (K-Ar) method, that allows dating of materials that are beyond the limit of radiocarbon dating (Table 1).
| Name of Method | Age Range of Application | Material Dated | Methodology |
Radiocarbon
| 1 - 70,000 years
| Organic material such as bones, wood, charcoal, shells
| Radioactive decay of 14C in organic matter after removal from bioshpere
|
| K-Ar dating | 1,000 - billion of years
| Potassium-bearing minerals and glasses
| Radioactive decay of 40K in rocks and minerals
|
Uranium-Lead
| 10,000 - billion of years
| Uranium-bearing minerals
| Radioactive decay of uranium to lead via two separate decay chains
|
Uranium series
| 1,000 - 500,000 years
| Uranium-bearing minerals, corals, shells, teeth, CaCO3
| Radioactive decay of 234U to 230Th
|
Fission track
| 1,000 - billion of years
| Uranium-bearing minerals and glasses
| Measurement of damage tracks in glass and minerals from the radioactive decay of 238U
|
Luminescence (optically or thermally stimulated)
| 1,000 - 1,000,000 years
| Quartz, feldspar, stone tools, pottery
| Burial or heating age based on the accumulation of radiation-induced damage to electron sitting in mineral lattices
|
Electron Spin Resonance (ESR)
| 1,000 - 3,000,000 years
| Uranium-bearing materials in which uranium has been absorbed from outside sources
| Burial age based on abundance of radiation-induced paramagnetic centers in mineral lattices
|
Cosmogenic Nuclides
| 1,000 - 5,000,000 years
| Typically quartz or olivine from volcanic or sedimentary rocks
| Radioactive decay of cosmic-ray generated nuclides in surficial environments
|
| Magnetostratigraphy | 20,000 - billion of years
| Sedimentary and volcanic rocks
| Measurement of ancient polarity of the earth's magnetic field recorded in a stratigraphic succession
|
Tephrochronology
| 100 - billions of years
| Volcanic ejecta
| Uses chemistry and age of volcanic deposits to establish links between distant stratigraphic successions
|
Table 1. Comparison of commonly used dating methods.
|
Radiation, which is a byproduct of radioactive decay, causes electrons to dislodge from their normal position in atoms and become trapped in imperfections in the crystal structure of the material. Dating methods like thermoluminescence, optical stimulating luminescence and electron spin resonance, measure the accumulation of electrons in these imperfections, or "traps," in the crystal structure of the material. If the amount of radiation to which an object is exposed remains constant, the amount of electrons trapped in the imperfections in the crystal structure of the material will be proportional to the age of the material. These methods are applicable to materials that are up to about 100,000 years old. However, once rocks or fossils become much older than that, all of the "traps" in the crystal structures become full and no more electrons can accumulate, even if they are dislodged.
Using paleomagnetism to date rocks and fossils
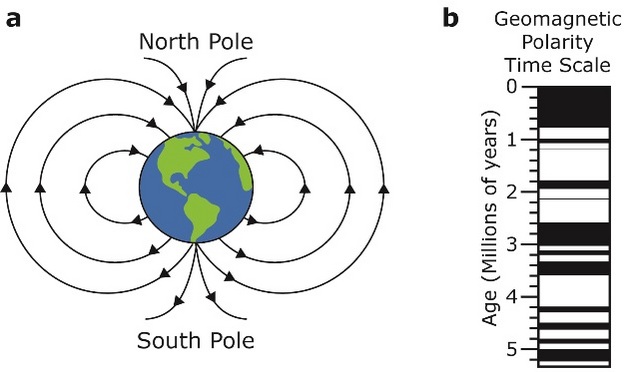
The Earth is like a gigantic magnet. It has a magnetic north and south pole and its magnetic field is everywhere (Figure 6a). Just as the magnetic needle in a compass will point toward magnetic north, small magnetic minerals that occur naturally in rocks point toward magnetic north, approximately parallel to the Earth's magnetic field. Because of this, magnetic minerals in rocks are excellent recorders of the orientation, or polarity, of the Earth's magnetic field.

Figure 6: The earth’s magnetic field can be measured to determine the polarity of a rock sample.
(a) The earth is surrounded by a magnetic field generated by the magnetism in the core of the earth. Small magnetic grains in rocks will orient themselves to be parallel to the direction of the magnetic field pointing towards the north pole. (b) The geomagnetic polarity time scale shows how the polarity of the earth’s magnetic field has changed through time. Black bands indicate times of normal polarity and white bands indicate times of reversed polarity.
© 2013
Nature Education All rights reserved.

Through geologic time, the polarity of the Earth's magnetic field has switched, causing reversals in polarity. The Earth's magnetic field is generated by electrical currents that are produced by convection in the Earth's core. During magnetic reversals, there are probably changes in convection in the Earth's core leading to changes in the magnetic field. The Earth's magnetic field has reversed many times during its history. When the magnetic north pole is close to the geographic north pole (as it is today), it is called normal polarity. Reversed polarity is when the magnetic "north" is near the geographic south pole. Using radiometric dates and measurements of the ancient magnetic polarity in volcanic and sedimentary rocks (termed paleomagnetism), geologists have been able to determine precisely when magnetic reversals occurred in the past. Combined observations of this type have led to the development of the geomagnetic polarity time scale (GPTS) (Figure 6b). The GPTS is divided into periods of normal polarity and reversed polarity.
Geologists can measure the paleomagnetism of rocks at a site to reveal its record of ancient magnetic reversals. Every reversal looks the same in the rock record, so other lines of evidence are needed to correlate the site to the GPTS. Information such as index fossils or radiometric dates can be used to correlate a particular paleomagnetic reversal to a known reversal in the GPTS. Once one reversal has been related to the GPTS, the numerical age of the entire sequence can be determined.
Summary
Using a variety of methods, geologists are able to determine the age of geological materials to answer the question: "how old is this fossil?" Relative dating methods are used to describe a sequence of events. These methods use the principles of stratigraphy to place events recorded in rocks from oldest to youngest. Absolute dating methods determine how much time has passed since rocks formed by measuring the radioactive decay of isotopes or the effects of radiation on the crystal structure of minerals. Paleomagnetism measures the ancient orientation of the Earth's magnetic field to help determine the age of rocks.
Glossary
absolute dating: Determining the number of years that have elapsed since an event occurred or the specific time when that event occurred
atomic mass: The mass of an isotope of an electron, based on the number of protons and neutrons
atomic nucleus: The assemblage of protons and neutrons at the core of an atom, containing almost all of the mass of the atom and its positive charge
daughter isotope: The isotope that forms as a result of radioactive decay
electrons: Negatively charged subatomic particles with very little mass; found outside the atomic nucleus
electron spin resonance: Method of measuring the change in the magnetic field, or spin, of atoms; the change in the spin of atoms is caused by the movement and accumulation of electrons from their normal position to positions in imperfections on the crystal structure of a mineral as a result of radiation.
elements: Chemical substances that cannot be split into a simpler substances
fault: A fracture in a rock along which movement occurs
geomagnetic polarity time scale: A record of the multiple episodes of reversals of the Earth's magnetic polarity that can be used to help determine the age of rocks
half-life: The amount of time it takes for half of the parent isotopes to radioactively decay to daughter isotopes
index fossil: A fossil that can be used to determine the age of the strata in which it is found and to help correlate between rock units
isotopes: Varieties of the same element that have the same number of protons, but different numbers of neutrons
magnetic field: A region where lines of force move electrically charged particles, such as around a magnet, through a wire conducting an electric current, or the magnetic lines of force surrounding the earth
magnetism: The force causing materials, particularly those made of iron and other certain metals, to attract or repel each other; a property of materials that responds to the presence of a magnetic field
normal polarity: Interval of time when the earth's magnetic field is oriented so that the magnetic north pole is approximately in the same position as the geographic north pole
neutrons: A subatomic particle found in the atomic nucleus with a neutral charge and a mass approximately equal to a proton
optical stimulating luminescence: Dating method that uses light to measure the amount of radioactivity accumulated by crystals in sand grains or bones since the time they were buried
paleomagnetism: Remanent magnetization in ancient rocks that records the orientation of the earth's magnetic field and can be used to determine the location of the magnetic poles and the latitude of the rocks at the time the rocks were formed
parent isotope: The atomic nucleus that undergoes radioactive decay
polarity (magnetic polarity): The direction of the earth's magnetic field, which can be normal polarity or reversed polarity
potassium-argon (K-Ar) method: Radiometric dating technique that uses the decay of 39K and 40Ar in potassium-bearing minerals to determine the absolute age
principle of cross-cutting relationships: Any geologic feature that cross-cuts across strata must have formed after the rocks they cut through were deposited.
principle of faunal succession: Fossil species succeed each other in a definitive, recognizable order and once a species goes extinct, it disappears and cannot reappear in younger rocks.
principle of original horizontality: Layers of strata are deposited horizontally, or nearly horizontally, and parallel or nearly parallel to the earth's surface.
principle of superposition: In an undeformed sequence, the oldest rocks are at the bottom and the youngest rocks are at the top.
protons: Positively charged subatomic particles found in the nucleus of an atom
radioactivity (radioactive): An unstable isotope spontaneously emits radiation from its atomic nucleus
radioactive decay: The process by which unstable isotopes transform to stable isotopes of the same or different elements by a change in the number of protons and neutrons in the atomic nucleus
radiocarbon dating: Radiometric dating technique that uses the decay of 14C in organic material, such as wood or bones, to determine the absolute age of the material
radiometric dating: Determination of the absolute age of rocks and minerals using certain radioactive isotopes
relative dating: Rocks and structures are placed into chronological order, establishing the age of one thing as older or younger than another
reversals (magnetic reversals): Changes in the earth's magnetic field from normal polarity to reversed polarity or vice versa
reversed polarity: Interval of time when the earth's magnetic field is oriented so that magnetic north pole is approximately in the same positions as the geographic south pole
strata (singular: stratum): Distinct layers of sediment that accumulated at the earth's surface.
stratigraphy: The study of strata and their relationships
thermoluminescence: Dating method that uses heat to measure the amount of radioactivity accumulated by a rock or stone tool since it was last heated